Study:
A 2-year prospective study (Jo et al., Arthroscopy, 2020) evaluated the use of autologous adipose-derived stem cells (AD-MSCs) for partial-thickness rotator cuff tears.
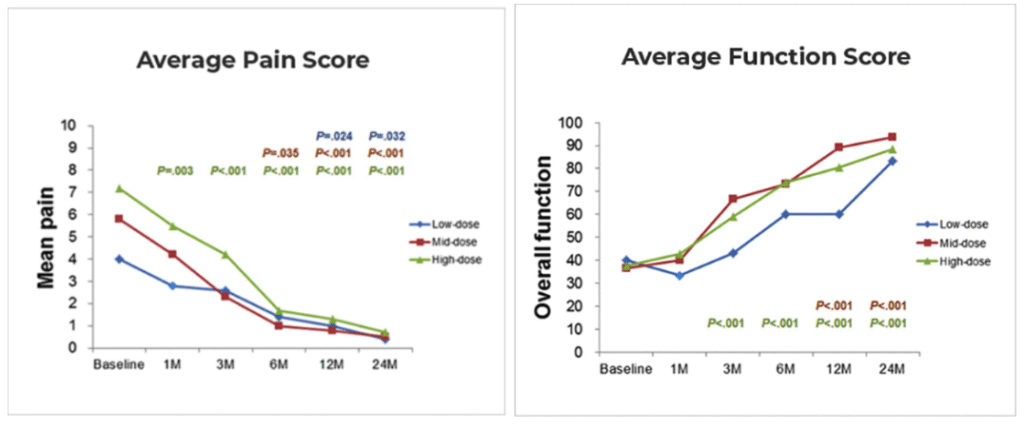
Nineteen patients received ultrasound-guided intratendinous injections of AD-MSCs. They were followed for 2-years and evaluated for pain, strength, function and tendon healing on MRI.
Results:
- Shoulder pain decreased by approximately 90% at both one and two years.
- Strength of the supraspinatus, infraspinatus, and teres minor increased
- Functional scores (ASES, Constant-Murley, SPADI) improved and remained stable through two years.
- MRI follow-up showed near-complete resolution of bursal-sided tendon defects by one year, with no recurrence at two years.
- None of the patients required surgical intervention or lost benefit during follow-up.
No treatment-related adverse events occurred during the 2-year follow-up.
Key Takeaway:
Intratendinous injection of autologous adipose-derived stem cells provides a safe, durable treatment option for partial-thickness rotator cuff tears. MRI follow-up confirmed tendon regeneration, while pain, strength, and function improvements were sustained for two years. This therapy represents a promising regenerative alternative for patients who do not respond to conservative care and seek an alternative to shoulder surgery.
At Integrative Rehab Medicine, our stem cell treatments are delivered under exact image guidance to target tendon and joint pathology with exceptional accuracy. We partner closely with local physical therapists to integrate healing with structured rehabilitation, supporting strength, motion, and durable functional recovery.
Reference:
Jo, C.H. et al., Arthroscopy: The Journal of Arthroscopic and Related Surgery (2020).
https://doi.org/10.1016/j.arthro.2019.11.120
